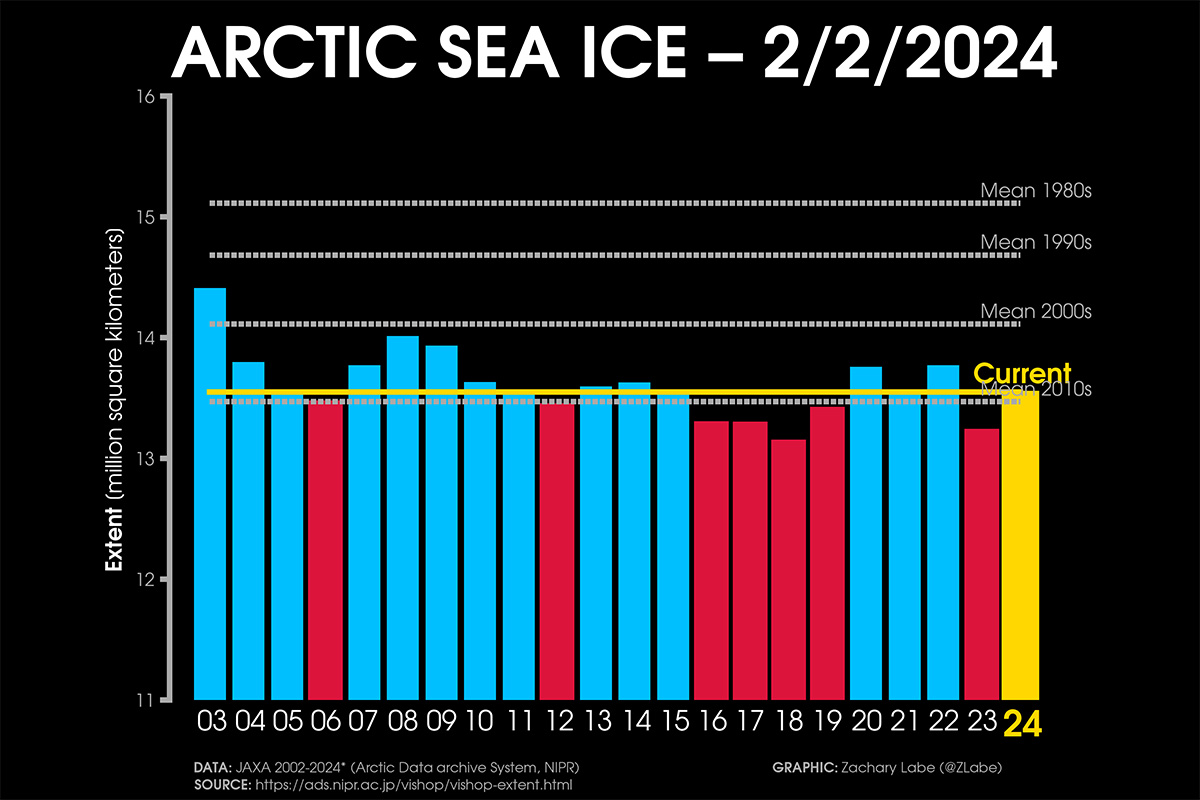
Arctic Sea Ice ranks as the third highest in the last 10 winters at the beginning of February and follows the normal growth of the 2010s
As winter unfolds, the Arctic sea ice takes center stage, showcasing a noteworthy ranking as the third-highest in the last 10 years. Nevertheless, weather patterns and sea surface temperatures might play an essential role during the melting season.
The Arctic sea ice extent when the last winter month started equals 14.141 million square kilometers, according to the US National Snow & Ice Data Center (NSIDC). The extent of sea ice changes daily due to the influence of weather patterns and strong winds. After being well higher the last 20-year extension, sea ice extent decreased in the last weeks due to the influence of intense storms affecting the Arctic.
Although much below the 1981-2010 average, sea ice extent is within the normal sea-ice inter-annual variability of the last 20 years, ranking as the third highest in the last 10 years, as depicted in the image below.
SEA ICE IN THE LONG-TERM CONTEXT
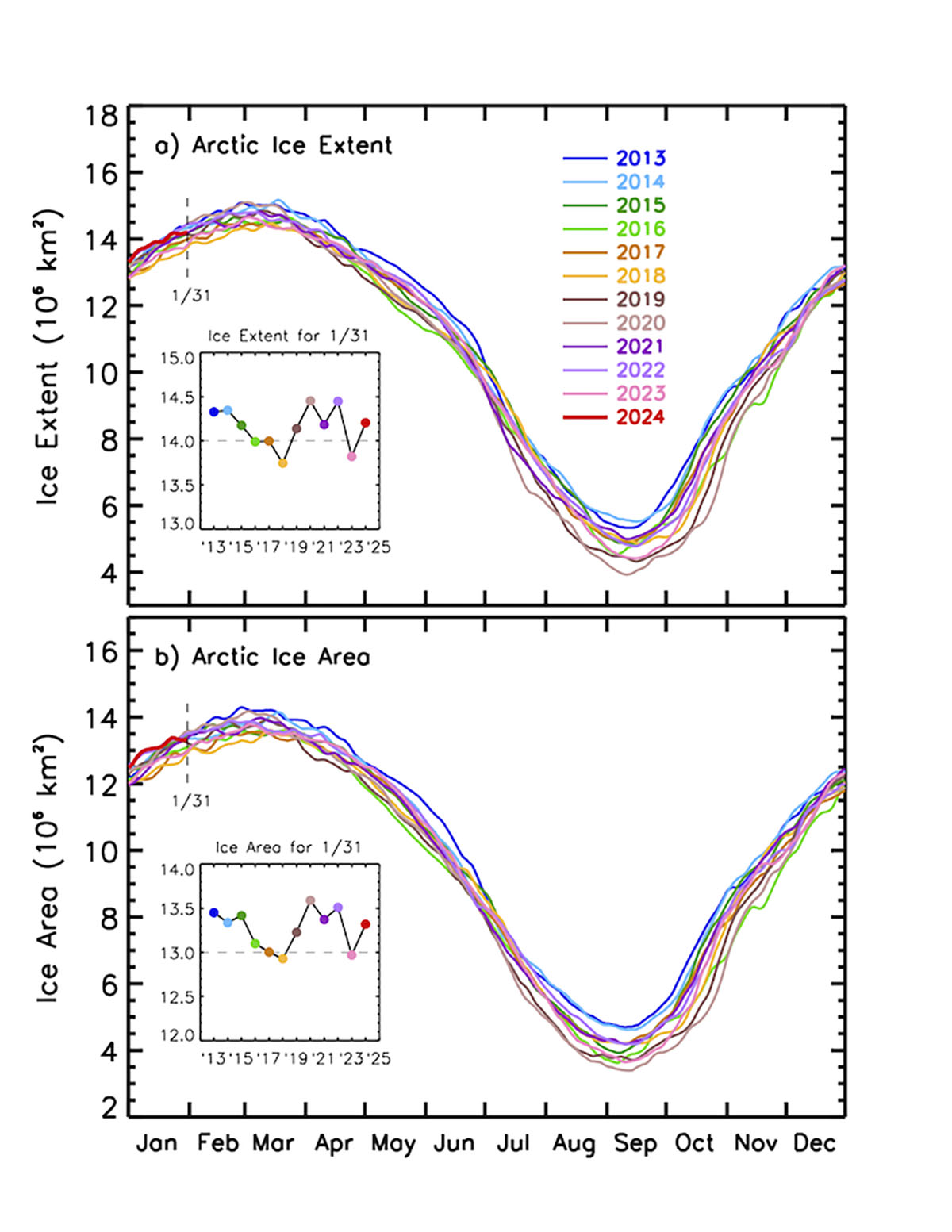
Through most of January, the 2024 sea ice extent was very high compared to the 2010s mean. At the beginning of January, it reached the average extent of the 2000s, setting the highest extension of the last 21 years.
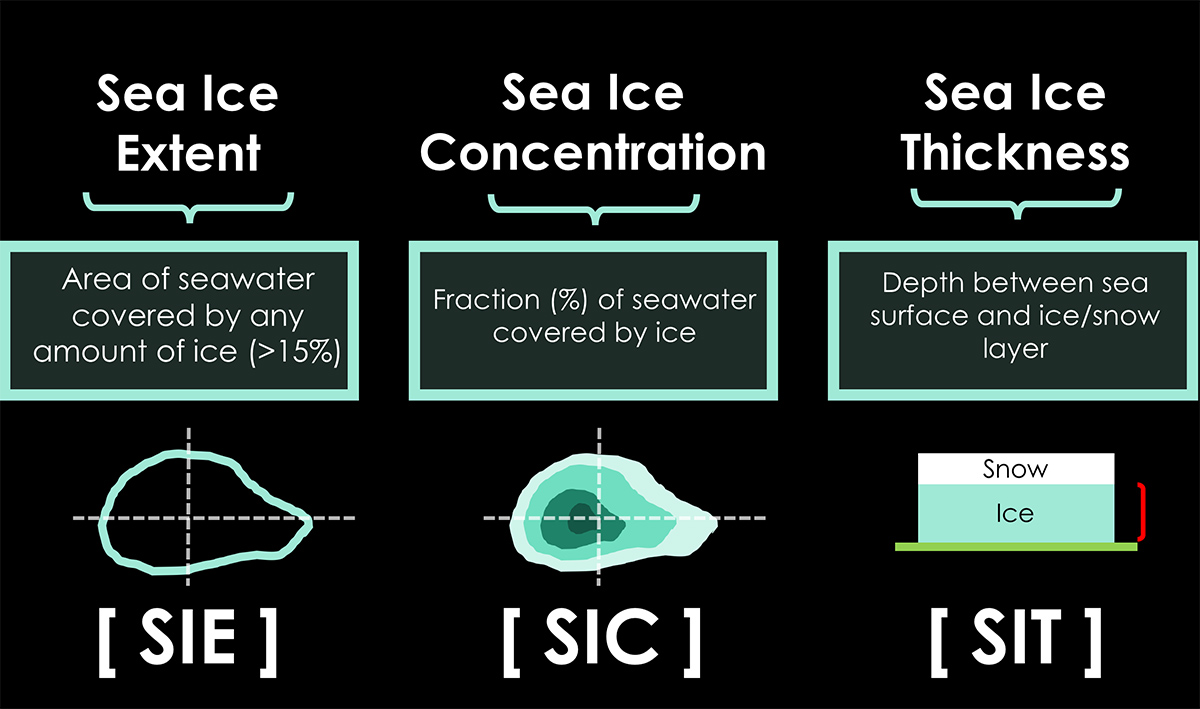
Sea ice extent defines the area of the ocean with at least 15% sea ice. The image below shows the sea ice extent on January 31st, 2024, compared with all years from 2012 on the Polar Portal.
The last week’s rapid shrinkage is visible and shows how fast the sea ice is moving north due to storms in the area. This shows the large natural daily variability in sea ice conditions, while the average extent represents an essential indicator of current weather and possible feedback in the upcoming Spring season.
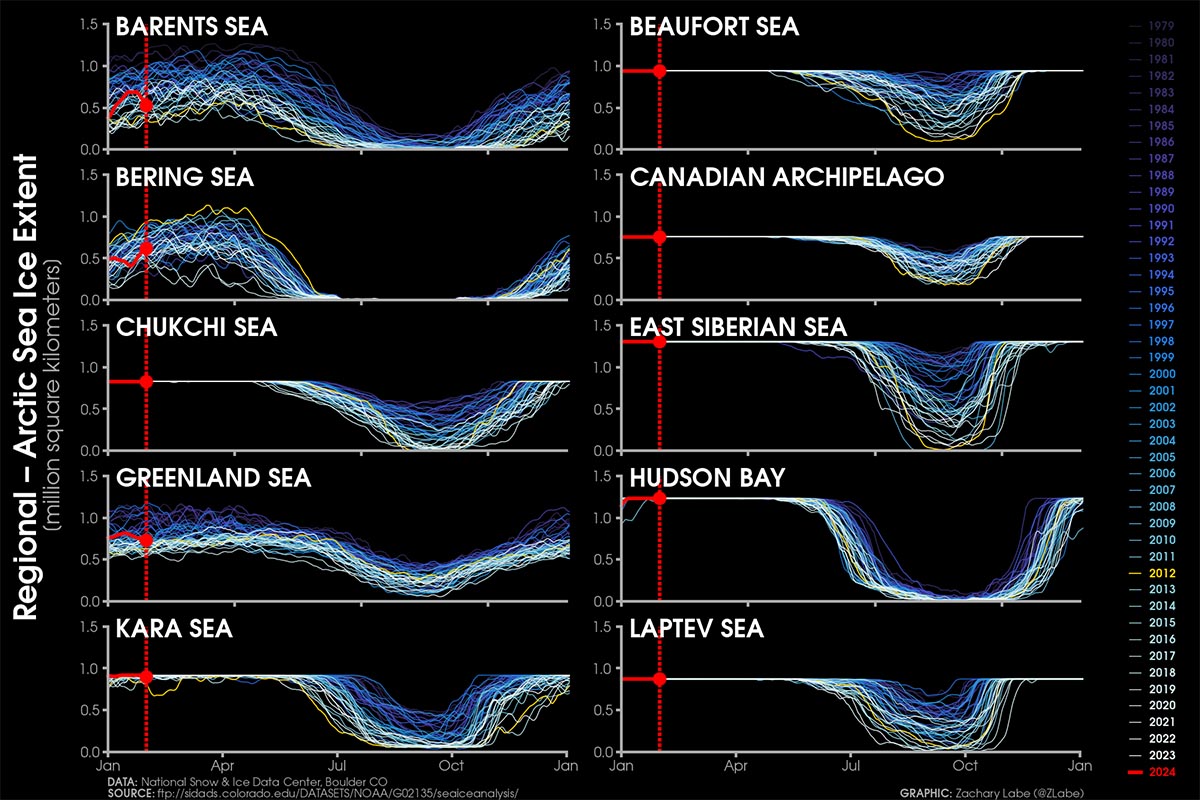
The excellent sea ice condition comes after an unusually high increase in December 2023. In December the Arctic sea ice averaged 12.00 million square kilometers, making it the ninth-lowest in the 45-year satellite record. The daily increase in sea ice extent averaged 87,400 square kilometers, surpassing the 1981 to 2010 average of 64,100 square kilometers per day.
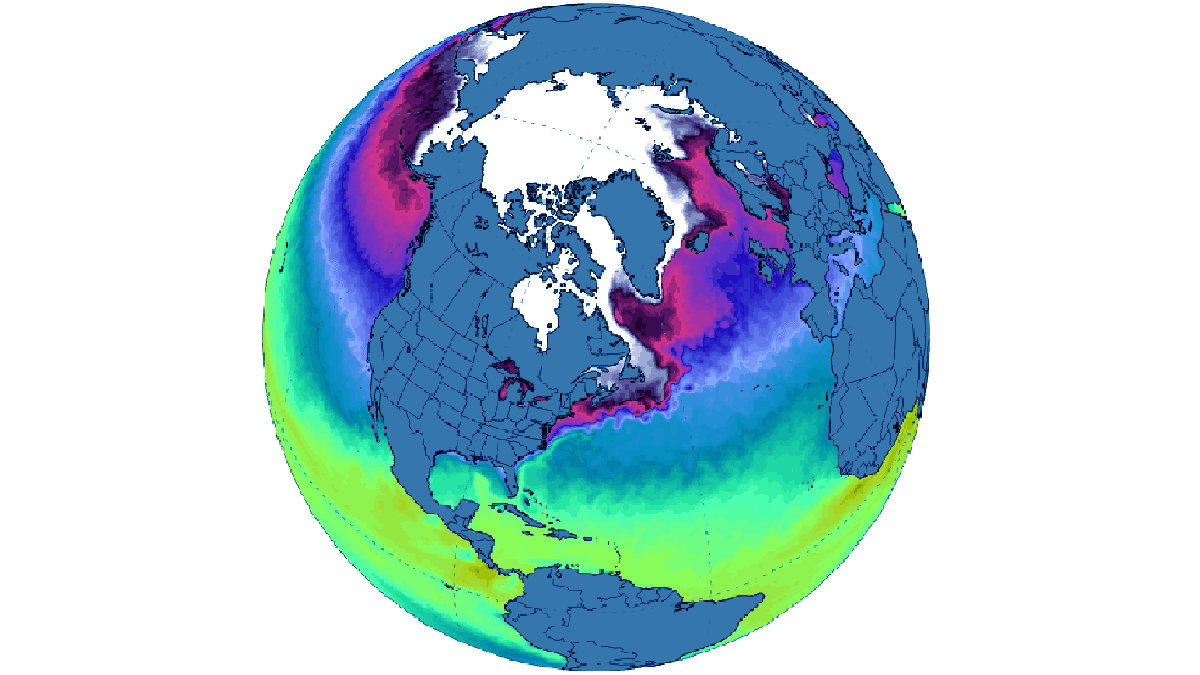
Despite a delayed start in the Hudson Bay freeze-up, ice formation progressed swiftly from west to east, leaving only a tiny open ocean area near the Belcher Islands by month’s end. Only in the northern Atlantic and the Barents Sea sea ice extent remained consistently below average, aligning with the decade-long trend. The image below comes from Zachary Labe
For December 2023, it registered as the third-highest monthly gain in the 45-year record, witnessing an increase of 2.71 million square kilometers. This places it behind 2006, which saw a gain of 2.85 million square kilometers, and 2016, with a gain of 2.78 million square kilometers.
Data comes from the Arctic Sea Ice news and analysis of the NSIDC. Below is the video of sea ice thickness and area in the Arctic in the last month, merging a few nice animations available from Zachary Labe and the Polar Portal
Daily evolution of sea ice and sea surface temperature in the Arctic
Analysis at the beginning of winter 2024 – SWE /RRC
ICE UNDER THE LENS
Ice plays a vital role in our planet’s energy system. The reason lies in the unique properties of ice crystals, which derive from the specific properties of water molecules, making them the key to life.
The basic structure of ice is a tetrahedron, and ice crystals preferentially grow along the axes of the basal plane rather than “vertically.” To understand the characteristics and behavior of sea ice, it is essential to emphasize that the “horizontal” planar direction is preferred in crystal growth when the ice surface thickens through freezing.
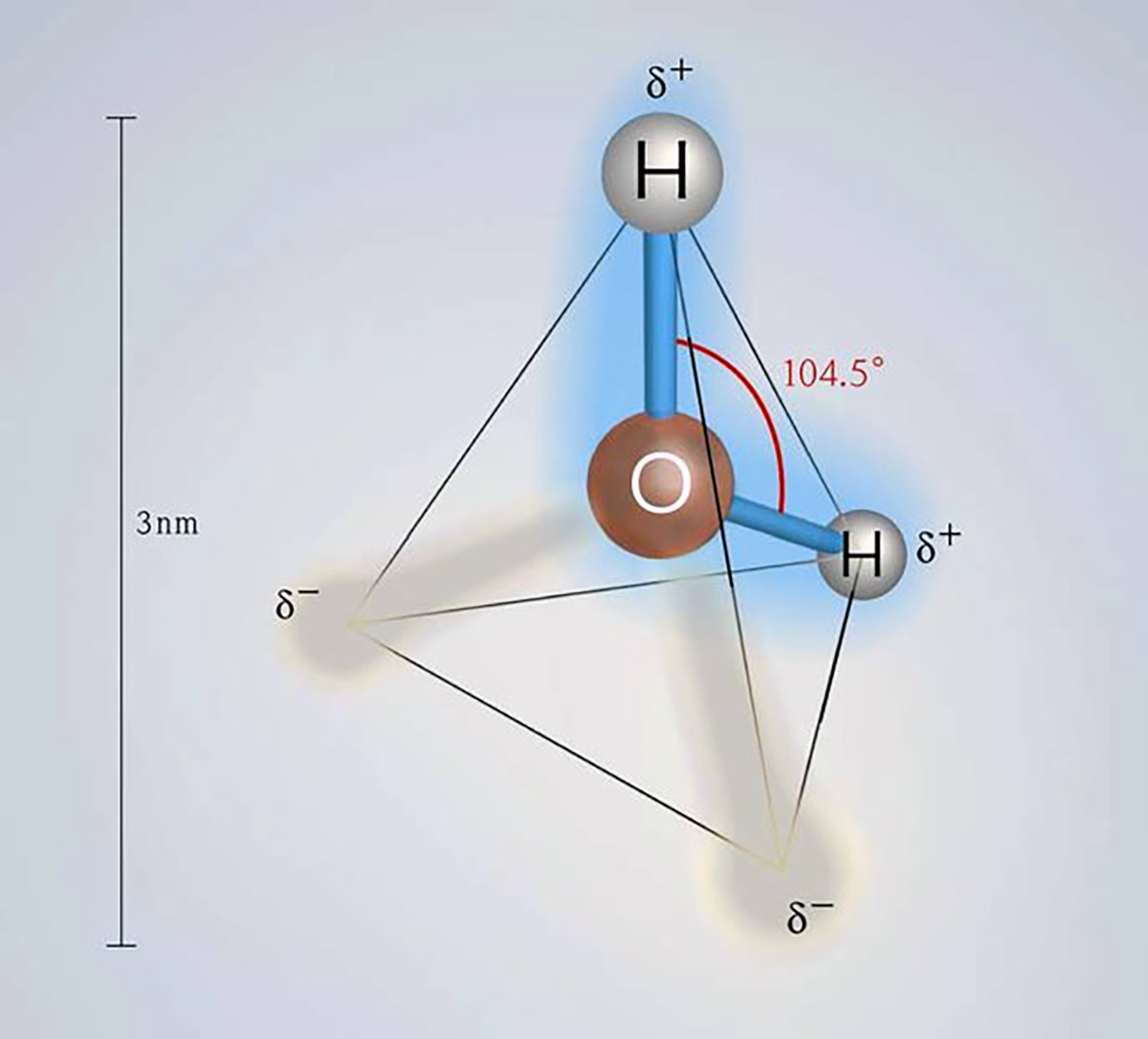
Image from Banik & Brückle (2010)
Water is an unusual molecule because the solid form is less dense than its liquid form, unlike metals. The density of water is 1000 kilograms per cubic meter (hence the definition of the kilogram). On the other hand, ice density is 917.4 kilograms per cubic meter.
Seawater has a higher density than pure water and usually equals 1025 kilograms per cubic meter. The density difference between water and ice is about 10%. This is why 10% of the mass of an ice sheet or iceberg emerges above the sea surface while the rest remains underwater.
Image from alearningfamily.com
Now, let’s consider what would happen if the density difference of water went in the opposite direction, as it does for most substances. In this case, ice would sink in water. Lakes, rivers, and even the sea would freeze, becoming almost solid. When formed on the surface of a body of water, for example, on the sea surface due to low temperatures, the ice would sink to the bottom and form a frozen layer there.
All benthic life forms would be eliminated. In the case of a lake, the ice layer at the bottom would increase until only a thin portion of unfrozen water remained on the surface by the end of the winter. Perhaps not even that. The entire life of the lake would be eliminated.

In the real world, sea ice forms a thin layer on the surface that protects the ocean from further freezing. Ice acts as an insulating layer between the atmosphere and the ocean. In the imaginary world where ice is denser than water, the marine surface would constantly be exposed to the atmosphere. It could cool without obstacles during winter, continuously absorbing its cold.
Ice skating would also be impossible if ice were denser than water. In the real world, the strong pressure of the skate blade on the ice surface lowers the melting point, and the ice just below it melts, lubricating it. If water were less dense, the pressure on the ice would raise the melting point, making ice skating impossible.
Image from travelalberta.com
Back to the real world. The maximum density of water is reached at around 4 degrees Celsius. This means that in autumn, a river or a lake located at high latitudes in contact with cold air temperatures will cool the surface water, causing it to sink initially to be replaced by warmer water from below. This happens because warm water is usually less dense than cold water, and this process is called convective overturning.
The phenomenon we just described continues until all the lake water is cooled to 4 degrees Celsius. At this point, the surface water can cool rapidly and reach 0 degrees Celsius, the freezing point of fresh water. However, the deeper parts of the lake remain at 4 degrees Celsius.
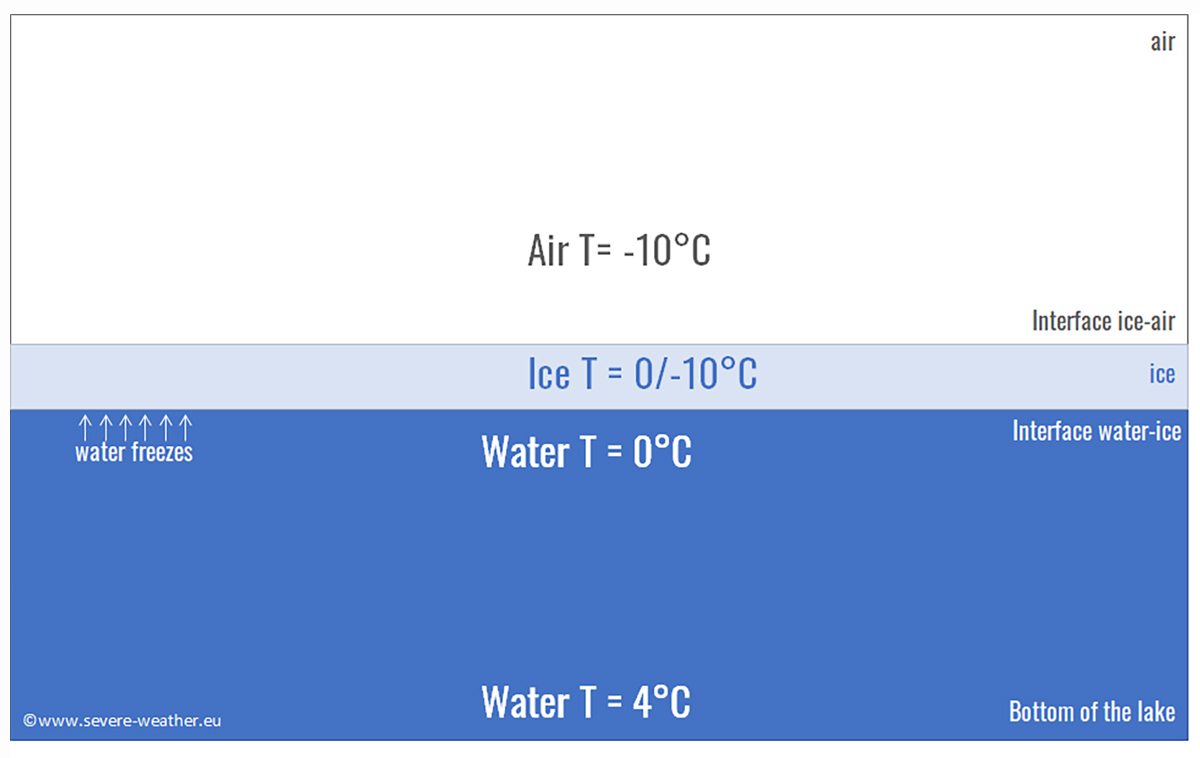
This is why the surface of a lake freezes quickly in autumn, while it takes much longer for the lake to freeze all the way to the bottom. In most cases, winter ends before this happens. However, seawater does not have this maximum density temperature. But what happens in the sea?
Seawater becomes denser as it cools until it reaches the freezing point. This transition in behavior from freshwater to saltwater occurs when the salinity exceeds 24.7 parts per thousand (ppt) of dissolved salt in water. Most seawater has a 32-35 ppt salinity, while only a few isolated seas have a salinity lower than 24.7 ppt. This occurs, for example, near the mouths of large Arctic rivers or the Baltic Sea. The generic term brackish defines this transition at 24.7 ppt.

sea ice forming around Svalbard
When seawater is cooled in autumn, convective overturning continues until all the water reaches freezing. The significant difference is that this freezing point is below 0 degrees Celsius because the presence of salt lowers it to -1.8 degrees Celsius for seawater. This effect is called cryoscopic lowering and is why salt is used on icy roads in temperate regions.
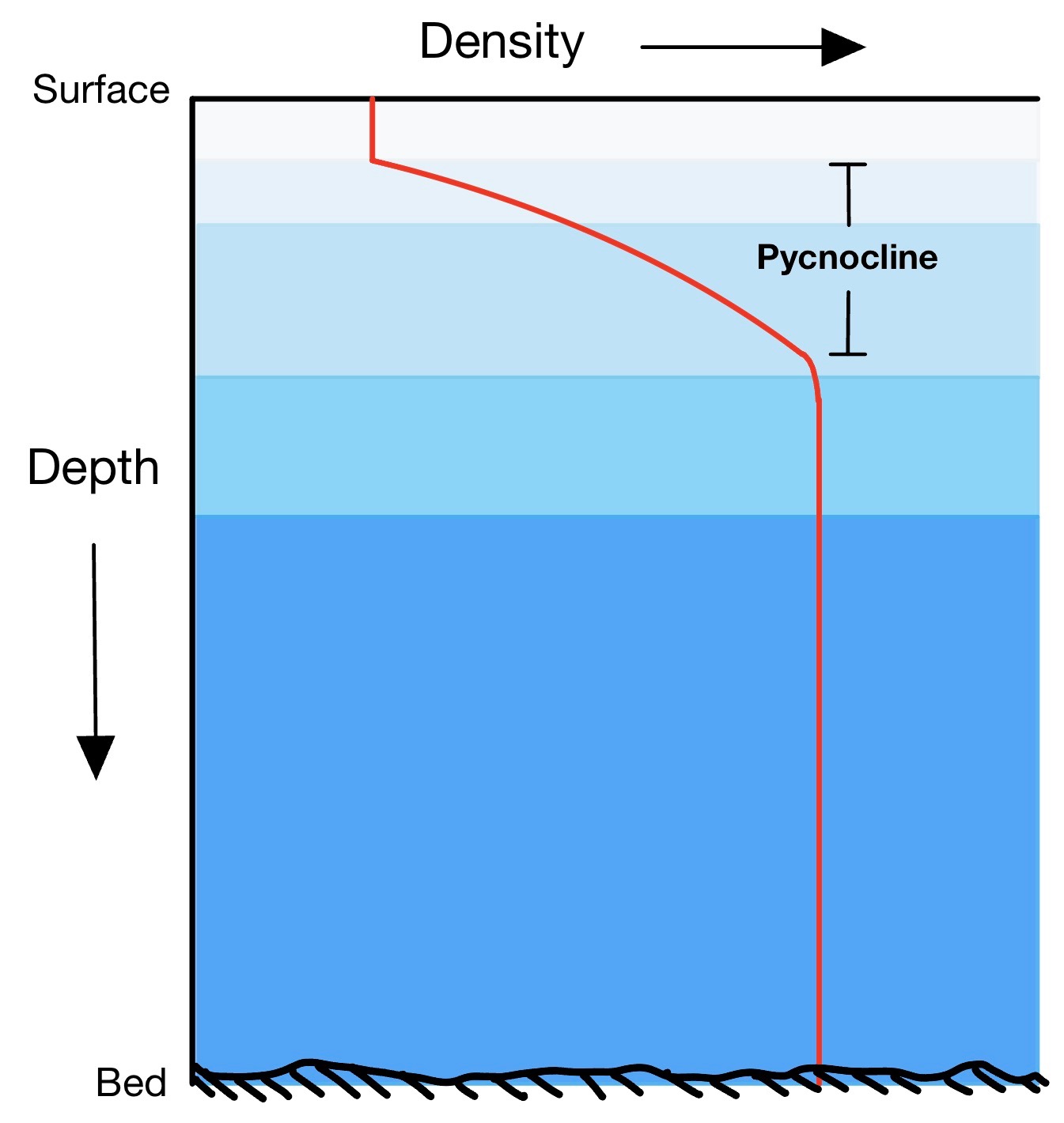
What prevents the entire depth of the ocean from cooling before freezing can occur on the surface is that the ocean consists of layers characterized by different types of water with different origins, moving in different directions and speeds. There is a rapid change in density between one layer and another, called the pycnocline. For this reason, convection must extend only to the bottom of the surface layer.
Image from wikipedia.com
In the Arctic, this layer is called Polar Surface Water, while the underlying layer is called Atlantic Water, as it reaches the Arctic from the Atlantic Ocean. The fact that ice floats on the water means that sea ice forms a thin cover on the sea surface, allowing ocean circulation to continue below and life to exist both in the depths of the ocean and, especially, near sea ice.
Another fundamental property of ice is its extraordinarily high latent heat of fusion. Latent heat is the heat required to melt ice when it is already at the melting point. This value is 80 kilocalories per kilogram.
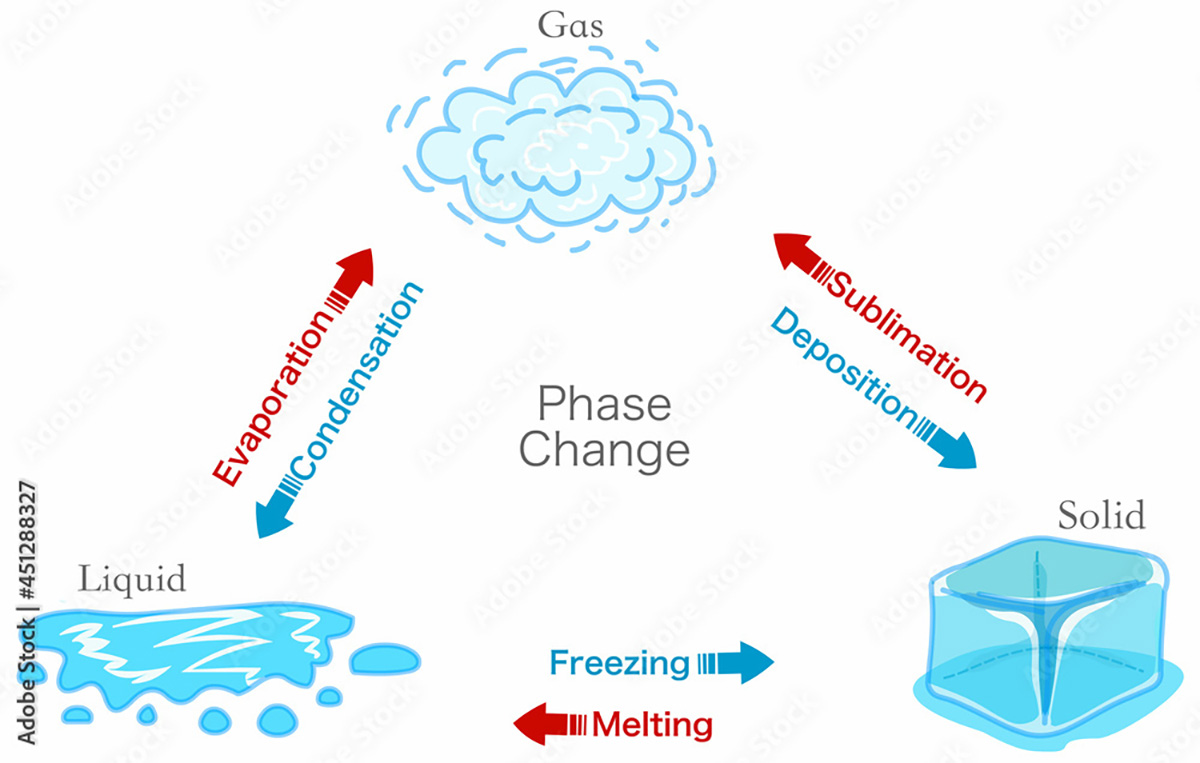
Phase change transition diagram. States matter schema. Evaporation, condensation, sublimation, deposition, freezing, melting, vaporization. Water, ice. Image from stock.adobe.com
Latent heat should not be confused with specific heat, defined as the heat required to raise the temperature of one kilogram of a substance by 1 degree Celsius. The specific heat of water is only 1 kilocalorie per kilogram, equal to 4186 joules.
This means that while only 1 kilocalorie of heat is needed to raise the temperature of 1 kilogram of ice by 1 degree Celsius, it is necessary to provide 80 kilocalories to melt it. In practice, the same amount needed to melt it could heat the same cold water mass to +80 degrees Celsius. The image below comes from Zachary Labe
The effect of Latent Heat of fusion maintains the air temperature near the surface at around 0°C during Summer
This aspect is of vital importance to Earth. On a planetary scale, the latent heat of fusion of water acts as a huge heat reservoir, a buffer for climate change. A fundamental example is the behavior of sea ice during the summer.
The ice melts, but as long as it does not melt completely, it maintains the air temperature near the surface and the temperature of the water under the ice at around 0 degrees Celsius. This happens because warmer air would be used to melt more ice, and it would cool itself during this process. As long as it is present in summer, sea ice provides a natural air and water conditioning system for the ocean.

Grease ice or frazil forming over the sea surface. Details in the following paragraph
HOW DOES SEAWATER FREEZES?
But how does seawater freeze? Let’s start by considering calm water that freezes in quiet conditions without waves. As the cold atmosphere extracts heat from the water’s surface, the surface molecules begin to freeze. This phenomenon creates a thin layer of independent ice crystals, initially as small disks and stars floating horizontally on the sea surface with a 2-3 millimeters diameter.
Each disk or star grows dendritically, branching out in 6 directions outward along the surface like a snowflake. These randomly shaped crystal pieces form a suspension that increases the surface density of the water. A kind of semi-liquid mixture is formed called frazil or grease ice, literally oily ice.

Nilas (young ice) are in their initial phase when it is almost transparent and dark. Svalbard, Norway
Under calm sea conditions, frazil crystals freeze by aggregating together to form a layer of young ice called nilas in its initial phases. When this nilas layer is only a few centimeters thick, it is transparent and is referred to as dark nilas. As it thickens, it first takes on a gray color and then becomes whitish, eventually turning opaque.
Once the nilas have formed, the sea can be considered physically separated from the atmosphere. At this point, a different type of growth begins. Water molecules start freezing by adhering to the bottom of the existing ice layer. This process is called freezing growth.
Both dark and opaque nilas coexist in the freezing process. Svalbard, Norway
After further growth, this process leads to the so-called ‘first-year ice,’ which reaches a thickness of about 1.5 meters in the Arctic in a single season and approximately 0.5-1 meter in Antarctica. What happens to the dissolved salt in seawater? Salt molecules cannot enter the crystalline structure, although some salt remains trapped in liquid pockets called brine cells.
For this reason, first-year ice is still slightly salty, with a salt concentration of about 10 ppm. The expulsion of brine from these cells occurs when the entire cell attempts to freeze as the temperature decreases. In the Arctic, only about half of the ice volume forms this way.
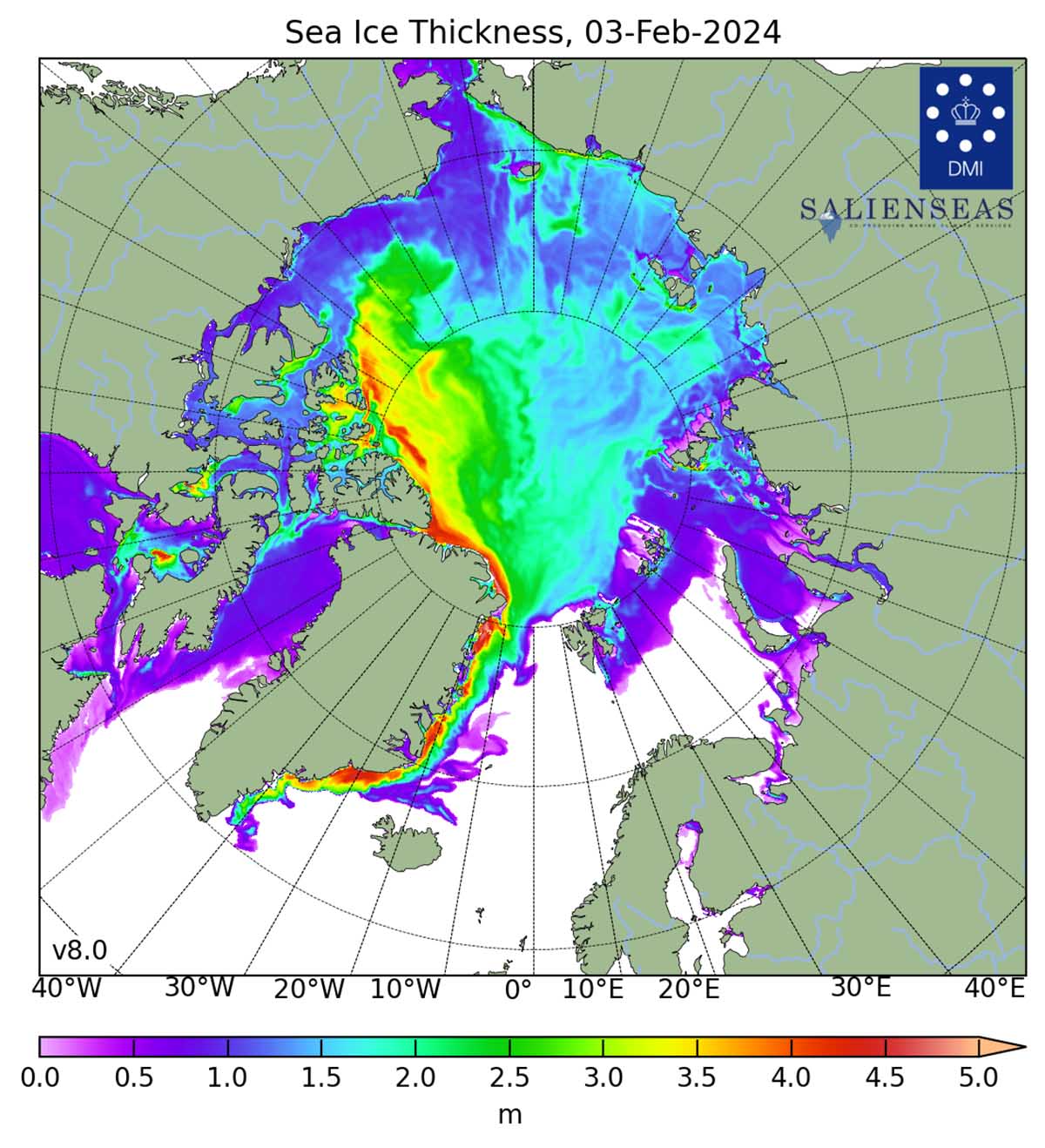
The map shows the sea ice thickness in the Northern Hemisphere, excluding the Baltic Sea and the Pacific. The data is based on Denmark Meteorological Institutes model calculations. The rest consists of deformations of pre-existing ice piled up in linear pressure ridges and openings created by this process, known as leads.
The ice layers formed through the processes described earlier are constantly in motion, guided by the frictional force of the wind on their surface and by marine currents on their underside. Compressive, divergent, and shear stresses continuously modify the Arctic sea ice.
The sea ice that forms in shallower waters or near the shore, where the atmosphere only needs to cool a thin layer of water to allow the surface to freeze, is called landfast or fast ice because it freezes the ocean to the seafloor. A bit farther out, the ice floats but remains stationary because it is attached to formations anchored on the seafloor.
Sea ice that is not fast refers to drift ice, and if the concentration exceeds 70%, it is called pack ice. When sea ice concentration is lower than 15%, this is considered open water, and the boundary between open water and ice is called the ice edge. Zachary Labe provides a nice sketch in the image below.
Sea ice cover in the Arctic grows throughout the winter and peaks in March. In September, the sea ice extension reaches its minimum, generally only around one-third of its winter maximum.
To get a proper picture of the sea ice state, there is a need to determine both extent and volume. Such numbers primarily include the ice thickness, generally linked to the ice age. Winter ice extent is generally a weaker indicator of what the ice extent will look like in September, when we will face the annual minimum.
SEA ICE AND THE WEATHER
Since 1979, it has been possible to monitor sea ice by satellite. We have 45 years of reliable information on the extent of the sea ice cover. The sea ice has continuously diminished, particularly since the end of the 1990s. Nevertheless, the winter trend is different from the summer trend.
The seasonal cycle of Arctic sea ice is characterized by the maximum annual extent in March, decreasing through spring and summer to an annual minimum extent in September. In the image below, the Arctic sea ice extent develops at the end of the winter season (March maximum) and by the end of the summer (September minimum). The image below comes from Zachary Labe
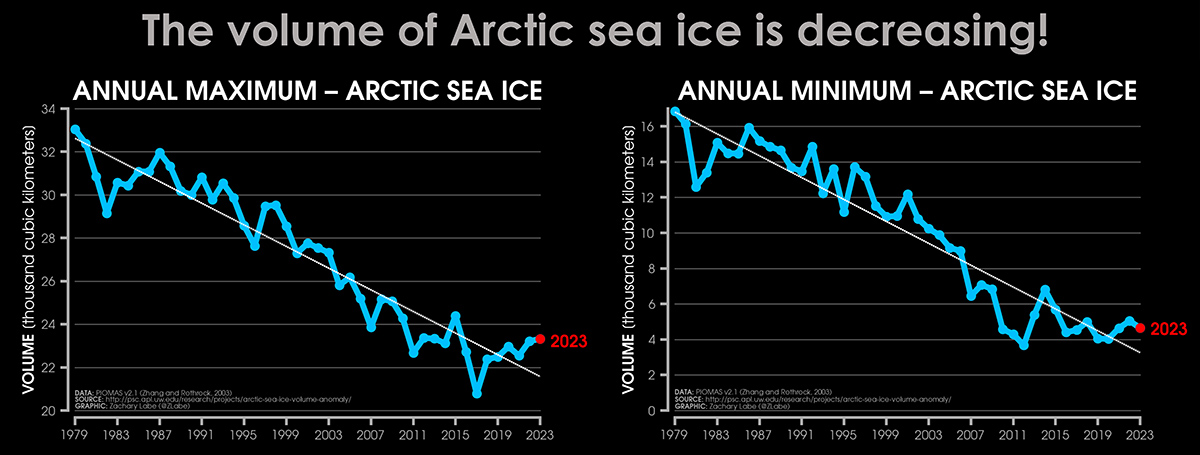
It is clear that in the last 6 years, after reaching a minimum in 2017, Arctic sea ice has been recovering its extent. Along with it, the sea ice volume has also increased and is now higher than in 2016.
This increased extent, which seems to be confirmed in 2024, can certainly influence the atmospheric patterns in the northern hemisphere during the upcoming spring. However, it should be noted that there have also been brief recovery trends in the past, such as from 2011 to 2015. Still, the long-term trend remains negative.
Moreover, It must be mentioned that ocean heat is the primary factor contributing to the early melting of Arctic sea ice. Specifically, the reduced temperature difference between the Arctic and the Tropics can lead to strong winds along the Gulf Stream. The combination of rising ocean heat and these strong winds can cause a sudden movement of heat towards the Arctic Ocean, resulting in a decline in sea ice extent.
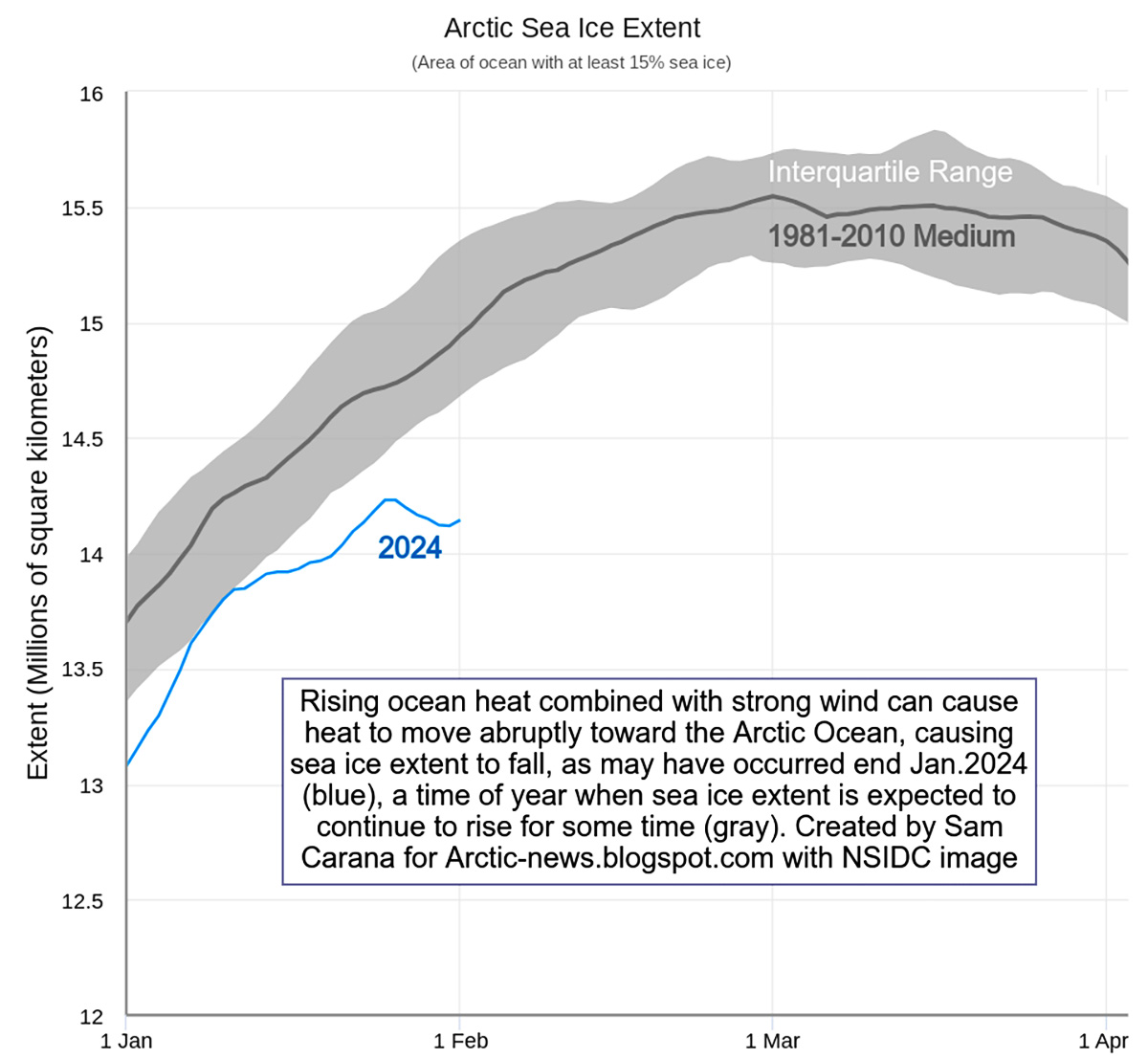
This phenomenon is exemplified by a drop in sea ice extent at the end of January 2024, when Arctic sea ice is typically expected to increase. The suggested cause for this anomaly is the potential influence of strong winds caused by the recent storm Ingunn, which transported a significant amount of ocean heat from the North Atlantic to the Arctic Ocean.
The information is supported by the image above depicting the observed decrease in sea ice extent during this period, which appeared these days on the Arctic News blog edited by Sam Carana.
We will keep you updated on this, so bookmark our page. Also, if you have seen this article in the Google App (Discover) feed or on social media, click the like button (♥) to see more of our forecasts and our latest articles on weather and nature in general.









I was recommended this website by my cousin I am not sure whether this post is written by him as nobody else know such detailed about my difficulty You are wonderful Thanks
Simply desire to say your article is as surprising The clearness in your post is simply excellent and i could assume you are an expert on this subject Fine with your permission let me to grab your feed to keep up to date with forthcoming post Thanks a million and please carry on the gratifying work